The Basics of Voltammetric Analysis
Voltammetry is a group of electroanalytical methods that measure current as a function of applied potential. This information is obtained under conditions that promote the polarization of a small indicator (working) electrode. When the current, which is proportional to the analyte concentration, is monitored at a fixed potential, the technique is called amperometry.
Key Concepts and Distinctions
- Polarization: In voltammetry, a small working electrode is used to ensure complete concentration polarization. This means that the rate of electrochemical reaction at the electrode surface is limited by the rate at which the analyte can diffuse or be transported to the surface. This is in contrast to:
- Potentiometry: Measurements are made at near-zero currents with no polarization. The electrode potential is a measure of the analyte’s activity/concentration in equilibrium with the electrode.
- Coulometry: Measures are taken to minimize or compensate for concentration polarization, and virtually all analyte is consumed. The total charge passed is measured, which is proportional to the total amount of analyte.
- Analyte Consumption: Voltammetry involves minimal consumption of the analyte, typically less than a few percent, making it a non-destructive or pseudo-nondestructive technique for the bulk solution. This is unlike coulometry where the analyte is largely converted to another state.
- Historical Context: The field developed from polarography, invented by Jaroslav Heyrovsky in the 1920s. Polarography specifically uses a dropping mercury electrode (DME) as the working electrode. While classical polarography’s importance has declined due to concerns about mercury toxicity and the advent of faster, more sensitive spectroscopic methods, the broader field of voltammetry and amperometry, employing a variety of other working electrodes, has grown significantly. Modern voltammetric techniques offer enhanced sensitivity, speed, and versatility.
Excitation Signals in Voltammetry
A variable potential excitation signal is applied to the working electrode, and the resulting current response is measured. The shape of this excitation signal determines the specific voltammetric technique. Four common waveforms are:
- Linear Scan (Figure 23-1a): In this method, the voltage applied to the working electrode increases (or decreases) linearly with time. The current flowing through the cell is recorded as a function of this applied voltage. This is the simplest form and is used in classical direct-current polarography and in basic hydrodynamic voltammetry setups. The resulting voltammogram is a sigmoidal wave.
- Pulse Excitation Signals (Figures 23-1b & 23-1c): These techniques apply potential pulses to the working electrode, and currents are measured at specific times during the pulse lifetime, often to minimize the impact of non-faradaic (charging) currents.
- Differential Pulse Voltammetry (Figure 23-1b): A small, fixed-magnitude potential pulse (e.g., 25-50 mV) is superimposed on a slowly increasing linear scan or staircase potential ramp. Two current measurements are made for each pulse: one just before the pulse (S1) and another at the end of the pulse (S2). The difference between these two currents (Δi=S2−S1) is recorded and plotted against the base potential. This yields a derivative-type curve with distinct peaks, significantly enhancing sensitivity and resolution compared to linear scan methods. The peak height is proportional to the analyte concentration.
- Square-Wave Voltammetry (Figure 23-1c): This technique applies a symmetrical square-wave pulse train superimposed on a staircase potential. It’s characterized by very fast scan rates (a complete voltammogram can be acquired in less than 10 milliseconds) and exceptionally high sensitivity. Current is typically measured twice during each cycle of the square wave: once at the end of the forward pulse and once at the end of the reverse pulse. The difference between these currents is plotted.
- Triangular Waveform (Figure 23-1d): In this method, the potential is scanned linearly from a starting potential to a switching potential, and then the scan direction is reversed, sweeping linearly back to the initial potential. This cyclic sweep is the basis of cyclic voltammetry (CV), which is particularly useful for studying the mechanisms of electrode reactions, identifying intermediates, and determining the reversibility of electrochemical processes.
Voltammetric Instrumentation
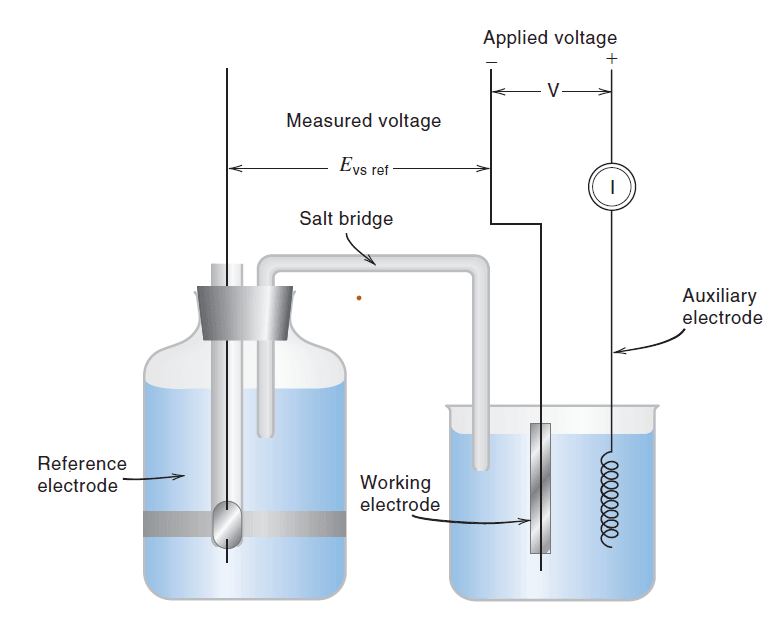
A typical voltammetric cell employs a three-electrode configuration to precisely control the potential of the working electrode and measure the current.
- Working Electrode (WE): This is the electrode at which the electrochemical reaction of interest (oxidation or reduction of the analyte) takes place. It typically has a small surface area (ranging from a few square millimeters down to micrometers or even nanometers) to facilitate rapid polarization and ensure that mass transport limits the current. Its potential is systematically varied against a reference electrode. Common materials include glassy carbon, platinum, gold, and mercury (in the form of a dropping mercury electrode or hanging mercury drop electrode).
- Reference Electrode (RE): This electrode maintains a constant and known potential throughout the experiment, regardless of the current flowing through the cell. It serves as a stable point of reference for measuring the potential of the working electrode. Common reference electrodes include the Saturated Calomel Electrode (SCE) and the Ag/AgCl electrode. Its placement is crucial for minimizing IR (ohmic) drop.
- Counter Electrode (CE) / Auxiliary Electrode: This electrode completes the electrical circuit and carries the current flowing from or to the working electrode. It is designed to have a large surface area so that its potential does not change significantly during the experiment. This prevents it from becoming polarized and affecting the working electrode potential. A platinum wire coil or a mercury pool are common choices.
- Supporting Electrolyte: An excess of a non-reactive inert salt (e.g., KCl, KNO₃, NaClO₄) is added to the analyte solution. Its primary functions are:
- Minimizing Migration: The high concentration of supporting electrolyte ions ensures that they carry the vast majority of the current through the solution, minimizing the contribution of analyte migration (movement under an electric field) to the overall mass transport. This makes diffusion and convection the dominant modes of analyte transport.
- Lowering Solution Resistance: A high concentration of ions significantly reduces the ohmic resistance (IR drop) of the solution, allowing for more accurate control of the potential at the working electrode.
Modern Instruments (Potentiostats)
Modern voltammetric instruments are highly sophisticated, employing electronics for precise generation of excitation signals and measurement of current. The heart of these instruments is typically a potentiostat.
- Potentiostat: This is an electronic device that automatically maintains the potential of the working electrode at a desired, controlled value relative to the reference electrode, even as the current in the cell changes. It does this by adjusting the voltage between the working electrode and the counter electrode. Operational amplifiers (op amps) are fundamental components within a potentiostat, used as current-to-voltage converters (to measure current) and in feedback loops to control the potential difference between the working and reference electrodes. A good potentiostat ensures accurate potential control despite changes in solution resistance or cell current.
Working Electrodes (Detailed)
The choice of working electrode is critical and depends on the specific analyte and the desired potential range.
- Common Solid Electrodes:
- Glassy Carbon: Widely used due to its wide potential range, low cost, good conductivity, chemical inertness, and mechanical strength. It is often polished to renew its surface.
- Carbon Paste Electrodes: Made from graphite powder mixed with a viscous organic liquid. They are easy to prepare and modify, offering a renewable surface.
- Gold, Copper, Nickel, Platinum: These metals are used when specific catalytic properties or potential windows are required. Platinum is particularly useful for oxidation reactions.
- Dropping Mercury Electrode (DME) (Figure 23-3e): Despite its decline due to mercury concerns, the DME was historically significant for polarography. Its advantages include:
- Reproducible, Fresh Surface: Each new mercury drop presents a clean, uncontaminated, and perfectly reproducible surface for electrochemical reactions. This minimizes problems associated with electrode fouling or surface contamination.
- High Overvoltage for H⁺ Reduction: Mercury has a high overvoltage for the reduction of hydrogen ions, meaning that hydrogen gas evolution is suppressed over a wide negative potential range. This allows for the study of many easily reducible organic and inorganic species in aqueous solutions that would otherwise be masked by hydrogen evolution.
- Formation of Amalgams: Many metal ions can be reversibly reduced and form amalgams with mercury, which can simplify their electrochemical behavior.
- Modified Electrodes: These are working electrodes whose surfaces have been intentionally altered by chemical modification (e.g., covalent bonding, adsorption, or polymer film deposition) to impart new or enhanced properties. Examples include:
- Electrocatalysis: Modifying the surface to catalyze specific electrochemical reactions, reducing overpotentials.
- Electrochromic Devices: Electrodes that change color upon electrochemical reaction.
- Selective Analytical Sensors: Designing electrodes with specific recognition elements for particular analytes, improving selectivity and sensitivity.
Voltammograms (Detailed Interpretation)
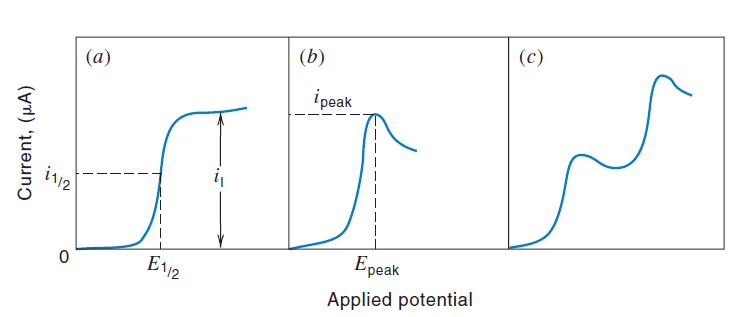
A voltammogram is the graphical representation of current response as a function of the applied potential. For a reduction process (cathodic current), it typically exhibits a sigmoidal shape, often referred to as a voltammetric wave.
- Residual Current: The small, non-faradaic current observed before the onset of the analyte’s electrochemical reaction. It arises from charging of the electrode-solution interface (capacitive current) and the reduction/oxidation of trace impurities in the supporting electrolyte. This current must be subtracted from the total current to obtain the true faradaic current due to the analyte.
- Onset Potential: The potential at which the current begins to significantly deviate from the residual current, indicating the start of the electrochemical reaction.
- Limiting Current (il): This is the constant current plateau observed at the top of the voltammetric wave. At potentials more negative (for reductions) or more positive (for oxidations) than the onset potential, the rate of mass transfer of the reactant to the electrode surface becomes the sole limiting factor for the current. This means that the analyte concentration at the electrode surface effectively drops to zero, and the current is then dictated by how quickly the analyte can be supplied from the bulk solution. The limiting current is directly proportional to the bulk analyte concentration: il=kcA, where k is a constant that depends on diffusion coefficient, electrode area, and diffusion layer thickness. This linearity is crucial for quantitative analysis.
- Half-wave Potential (E1/2): This is the potential at which the current reaches exactly half of the limiting current (i=il/2). For reversible electrochemical reactions, the half-wave potential is closely related to the standard electrode potential (E0) of the redox couple. It serves as a valuable qualitative identifier for the species undergoing reaction, as it is characteristic of a particular redox system under specific solution conditions (pH, supporting electrolyte).
- Anodic Waves: These are observed for oxidation reactions and typically appear at more positive potentials. The current will increase as the potential becomes more positive.
- Mixed Anodic/Cathodic Waves: For reversible redox couples, a complete cyclic voltammogram will show both a cathodic wave (for reduction) and an anodic wave (for oxidation).
- Mixtures: When a solution contains multiple electroactive species, the total voltammogram is typically the sum of the individual waves for each species. Quantitative determination of multiple species is possible if their half-wave potentials are sufficiently separated. Generally, a difference of 0.1-0.2 V is needed for two-electron reductions, while about 0.3 V separation is required for one-electron reductions to allow for clear resolution of the waves.
Hydrodynamic Voltammetry
In hydrodynamic voltammetry, the analyte solution or the working electrode is kept in continuous and reproducible motion (e.g., by stirring, rotating the electrode, or flowing the solution). This controlled motion enhances and stabilizes the mass transport of the analyte to the electrode surface, leading to reproducible and stable limiting currents rapidly.
- Mass Transport Mechanisms: Reactant species are transported from the bulk solution to the electrode surface by three primary mechanisms:
- Migration: The movement of charged ions under the influence of an electric field. In voltammetry, this is minimized by adding a high concentration of supporting electrolyte, which carries almost all of the current.
- Convection: The transport of species due to mechanical stirring, forced flow of the solution, or agitation caused by temperature or density gradients. In hydrodynamic methods, convection is deliberately controlled and used to enhance mass transport.
- Diffusion: The movement of species from a region of higher concentration to a region of lower concentration, driven by the concentration gradient. This is the dominant mode of mass transport in the immediate vicinity of the electrode surface.
- Nernst Diffusion Layer (δ): When convection is present, a thin, stationary (or stagnant) layer of solution exists immediately adjacent to the electrode surface. This is known as the Nernst diffusion layer. Within this layer (typically 10⁻² to 10⁻³ cm thick), mass transport occurs exclusively by diffusion because the solution flow is negligible near the surface. Outside this layer, convection rapidly replenishes the bulk concentration of the analyte. The thickness of this layer is inversely related to the rate of stirring or rotation; faster stirring leads to a thinner diffusion layer and thus higher limiting currents.
- Current/Voltage Relationships (Reversible Reactions):
- The limiting current (il) in hydrodynamic voltammetry is directly proportional to the analyte concentration and inversely proportional to the diffusion layer thickness (d or δ). The equation provided (il=dnFADAcA=kAcA, Equation 23-6) highlights this relationship, where n is the number of moles of electrons, F is the Faraday constant, A is the electrode area, and DA is the diffusion coefficient of analyte A.
- For a reversible reaction, the shape of the voltammetric wave can be described by the equation: Eappl=E1/2−n0.0592logil−ii (Equation 23-13). This equation allows for the determination of n from the slope of a plot of Eappl versus logil−ii.
- Irreversible Reactions: In contrast to reversible reactions, irreversible electrochemical reactions result in waves that are more drawn-out and less well-defined. The half-wave potentials for irreversible reactions may show a dependence on concentration and scan rate. However, the diffusion currents still remain linearly related to the bulk analyte concentration, making these reactions suitable for quantitative analysis, typically by using calibration standards.
- Rotating Disk Electrode (RDE): A common working electrode in hydrodynamic voltammetry where a disk-shaped electrode is embedded in an insulating material and rotated at a precisely controlled, constant high speed. The rotation creates a well-defined and reproducible flow pattern (laminar flow) towards the electrode surface, leading to a predictable and uniform diffusion layer. The limiting current for an RDE follows the Levich equation: il=0.620nFAD2/3ν−1/6cA (Equation 23-15), where ν is the kinematic viscosity of the solution and ω is the angular rotation rate. The RDE is invaluable for fundamental studies of reaction kinetics and mechanisms, as a plot of il vs. ω1/2 (a Levich plot) should be linear and can provide kinetic parameters.
- Rotating Ring-Disk Electrode (RRDE): This is an advanced version of the RDE, featuring a second, concentric ring-shaped electrode around the central disk electrode, separated by a thin insulating gap. This configuration allows for the detection of reaction intermediates generated at the disk electrode as they flow radially outward and are detected (oxidized or reduced) at the ring electrode. It’s exceptionally useful for probing complex electrode reaction mechanisms and identifying unstable intermediates.
Amperometric Titrations
Amperometric titrations utilize hydrodynamic voltammetry to detect the endpoint of a titration. Instead of monitoring potential changes (as in potentiometric titrations), the current is measured at a fixed potential (typically within the limiting current region of one or more species) as a function of the volume of reagent added.
- Endpoint Detection: Plots of current versus the volume of titrant added yield straight line segments with different slopes before and after the equivalence point. The intersection of these extrapolated lines indicates the endpoint. This method is effective even when the reaction product is insoluble or precipitates, unlike some other detection methods.
- Types of Curves (Figure 23-18):
- Analyte reacts, reagent does not: The current decreases as the analyte is consumed, then becomes constant after the endpoint.
- Reagent reacts, analyte does not: The current remains constant until the endpoint, then increases as excess reagent is added.
- Both analyte and reagent react: The current changes before and after the endpoint, creating a V-shaped or inverted V-shaped curve.
- Electrodes: Often employs a rotating platinum electrode (RPE) or twin platinum electrodes (for biamperometric titrations).
- Applications: Commonly used in automated instruments for specific analyses, such as chloride determination using coulometrically generated silver ions, or the Karl Fischer titration for precise water content determination.
Polarography
Polarography is a specific type of voltammetry that uses a dropping mercury electrode (DME) as the working electrode. It is distinguished from hydrodynamic voltammetry by the absence of forced convection or intentional stirring; mass transport occurs primarily by diffusion.
- Polarographic Currents: Due to the periodic growth and dislodgment of mercury drops, the current observed in polarography exhibits characteristic periodic fluctuations (Figure 23-21). The current increases as the drop grows (increasing surface area) and then sharply decreases when the drop falls off and a new one begins to form. The diffusion current (id) is typically taken as the difference between the average (or maximum) limiting current and the residual current.
- Ilkovic Equation: This fundamental equation quantitatively relates the average (or maximum) polarographic diffusion current to various experimental variables: (id)avg=607nD1/2m2/3t1/6c (for average current) (id)max=708nD1/2m2/3t1/6c (for maximum current, Equation 23-16) where n is the number of electrons, D is the diffusion coefficient of the analyte, m is the mass flow rate of mercury through the capillary (mg/s), t is the drop time (s), and c is the analyte concentration (mmol/L). This equation is vital for theoretical understanding and quantitative applications.
- Residual Current (Figure 23-22): Even in the absence of electroactive species, a small current flows through the polarographic cell. This residual current has two main components:
- Faradaic Current from Impurities: Due to the reduction or oxidation of trace impurities present in the supporting electrolyte or solvent. Careful purification of reagents is essential to minimize this.
- Charging (Capacitive) Current: This is a non-faradaic current that arises from the charging of the electrical double layer at the surface of the growing mercury droplet. As the surface area of the drop expands, ions in the solution rearrange to form an electrical double layer, requiring a transient current. This charging current changes with potential and drop size. It is a major limiting factor for the sensitivity of classical polarography, as it can be significant compared to the faradaic current from very dilute analytes.
- Decline in Importance: Classical direct-current polarography has largely been superseded by more advanced voltammetric techniques. Reasons for its decline include:
- Mercury Usage: Environmental and health concerns related to mercury.
- Cumbersome Apparatus: The need for precise control of the dropping mercury flow and drop time.
- Limited Sensitivity: Detection limits are typically around 10⁻⁵ M, which is not competitive with modern spectroscopic methods or pulse voltammetry techniques.
- Charging Current Interference: The significant charging current often masks the small faradaic currents from dilute analytes.
Cyclic Voltammetry (CV)
Cyclic voltammetry is one of the most widely used voltammetric techniques for fundamental studies of electrochemical processes. It uses a triangular voltage waveform (Figure 23-23) applied to a stationary working electrode in an unstirred solution. The potential is scanned linearly from a starting potential to a switching potential, and then the scan direction is reversed, sweeping linearly back to the initial potential.
- Electrodes: Small, solid, stationary working electrodes are typically used (e.g., polished platinum, glassy carbon, carbon paste, gold). The solution is unstirred to ensure that mass transport is diffusion-controlled.
- Cyclic Voltammogram (Figure 23-24): The resulting plot of current versus applied potential shows distinct peaks corresponding to the oxidation and reduction of the electroactive species.
- Cathodic Peak (Reduction): Appears during the forward scan (e.g., negative-going scan for a reduction). Characterized by a cathodic peak potential (Epc) and a cathodic peak current (ipc).
- Anodic Peak (Oxidation): Appears during the reverse scan (e.g., positive-going scan for an oxidation). Characterized by an anodic peak potential (Epa) and an anodic peak current (ipa).
- Characterizing Reversible Reactions: For a chemically reversible and electrochemically reversible (Nernstian) reaction:
- Peak Currents: The magnitudes of the cathodic and anodic peak currents are approximately equal: ipc≈∣ipa∣.
- Peak Separation (ΔEp=∣Epa−Epc∣): The difference between the anodic and cathodic peak potentials is characteristic of the number of electrons transferred. For a reversible reaction at 25°C, ΔEp=n0.0592 V (Equation 23-17), where n is the number of electrons involved in the redox process. Deviations from this value typically indicate an irreversible or quasi-reversible reaction.
- Peak Potential and Half-Wave Potential: The mid-point between the anodic and cathodic peak potentials, (Epa+Epc)/2, is approximately equal to the half-wave potential (E1/2) and also close to the formal standard potential (E^0′) for a reversible system.
- Randles-Sevcik Equation: For a reversible electrochemical process, the peak current is related to various parameters by the Randles-Sevcik equation: ip=(2.686×105)n3/2AD1/2Cν1/2 (Equation 23-18) where ip is the peak current (A), n is the number of electrons, A is the electrode area (cm²), D is the diffusion coefficient (cm²/s), C is the bulk concentration (mol/cm³), and ν is the scan rate (V/s). This equation shows that the peak current is proportional to the square root of the scan rate and directly proportional to the concentration.
- Fundamental Studies: The primary utility of cyclic voltammetry lies in its ability to provide qualitative and diagnostic information about electrochemical processes. It can:
- Determine Reaction Mechanisms: By observing the shapes of the peaks, their potential separation, and their dependence on scan rate, one can deduce whether a reaction is reversible, irreversible, or quasi-reversible, and if coupled chemical reactions are involved.
- Detect Intermediates: For example, in the reduction of parathion (Figure 23-25), the appearance of new peaks on the reverse scan can indicate the formation of unstable intermediates that are then oxidized.
- Identify Redox Couples: The characteristic peak potentials help identify the redox species present in a solution.
- Quantitative Analysis: While not as common for routine quantitative analysis as other voltammetric methods due to potential complications from background currents and peak broadening, peak currents are directly proportional to analyte concentration, and CV is increasingly applied for quantitative purposes, especially in research settings.
Pulse Voltammetry
Pulse voltammetry techniques were developed to significantly improve the detection limits and speed of electrochemical measurements compared to classical linear-scan voltammetry. They achieve this by strategically measuring the faradaic current at specific times during potential pulses, thereby minimizing the contribution of the non-faradaic (charging) current.
Differential-Pulse Voltammetry (DPV)
Differential-pulse voltammetry is highly sensitive and widely used for trace analysis.
- Excitation Signals (Figure 23-26):
- Analog Instruments: A small amplitude potential pulse (e.g., 25 or 50 mV) is superimposed on a slow, linear potential ramp.
- Digital Instruments: The pulse is superimposed on a staircase potential signal, where the potential increases in discrete steps.
- Current Measurement: For each pulse, two current measurements are made:
- S1: A measurement taken just before the application of the pulse (at the initial potential of the step).
- S2: A measurement taken at the end of the pulse (typically just before the potential returns to the base value). The difference between these two currents, Δi=S2−S1, is recorded and plotted against the average of the base potential for each pulse.
- Voltammogram (Figure 23-27): The resulting voltammogram is a derivative-type curve, meaning it appears as a peak rather than a sigmoidal wave.
- Peak Height: The height of the peak is directly proportional to the analyte concentration, which makes it suitable for quantitative analysis.
- Peak Potential: The potential at the maximum of the peak is approximately equal to the half-wave potential (E1/2) for a reversible reaction and close to the standard potential (E^0′).
- Advantages:
- Enhanced Resolution: DPV can resolve waves with half-wave potentials that differ by as little as 0.04-0.05 V (for two-electron reductions), significantly better than classical polarography.
- Increased Sensitivity: DPV offers detection limits that are 2 to 3 orders of magnitude lower than classical polarography, typically in the 10⁻⁷ to 10⁻⁸ M range. This improved sensitivity stems from the differential measurement, which effectively subtracts out most of the non-faradaic charging current, leaving primarily the faradaic current. The charging current decays much faster than the faradaic current, so measuring at the end of the pulse minimizes its contribution.
- Applications: Widely used for the trace determination of heavy metal ions (e.g., lead, cadmium, copper, zinc) in environmental samples, as well as for various organic compounds.
Square-Wave Voltammetry (SWV)
Square-wave voltammetry is a very fast and highly sensitive pulse technique.
- Excitation Signal (Figure 23-28): It involves a symmetrical square-wave pulse train superimposed on a staircase potential ramp. The frequency of the square wave can be high (e.g., 100-200 Hz).
- Speed & Sensitivity: SWV is known for its remarkable speed, capable of acquiring a complete voltammogram in less than 10 milliseconds. Its detection limits are comparable to or even better than DPV, often reaching 10⁻⁷ to 10⁻⁸ M.
- Current Measurement: The current is measured twice during each square-wave cycle:
- i1: At the end of the forward pulse (relative to the base potential).
- i2: At the end of the reverse pulse. The difference between these two currents, Δi=i1−i2, is plotted against the average of the potential step. This differential measurement, combined with the high scan rate, leads to a significant cancellation of the charging current, thus maximizing the signal-to-noise ratio.
- Voltammogram: Similar to DPV, the resulting voltammogram is a peak-shaped curve, with the peak height proportional to concentration and the peak potential providing qualitative information.
- Applications: SWV is routinely used with various working electrodes, including hanging mercury drop electrodes, solid microelectrodes, and chemically modified electrodes. It finds extensive applications in the analysis of inorganic and organic species, and as a highly sensitive detector in separation techniques like liquid chromatography (LC-SWV). Signal averaging from multiple rapid scans can further improve the precision of measurements.
Applications of Voltammetry
Voltammetric methods are primarily used for quantitative analysis, relying on the direct proportionality between the limiting current (or peak current in pulse techniques) and the analyte concentration.
- Calibration Curves: The most common approach involves generating a calibration curve by plotting the measured current (limiting current, peak height) against the known concentrations of a series of standard solutions. The concentration of an unknown sample is then determined by interpolating its measured current on this curve.
- Standard-Addition Methods: This method is preferred when the sample matrix is complex or when matrix effects might influence the current response. Known amounts of the analyte standard are added to the unknown sample, and the current is measured after each addition. Plotting current vs. added concentration allows for extrapolation to determine the original concentration.
- Matrix Matching: Accurate quantitative results necessitate careful matching of standards and samples regarding their electrolyte concentrations, pH, and other matrix components to ensure similar mass transport conditions and minimize interferences.
- Precision and Accuracy: Voltammetric methods typically achieve relative precisions and accuracies in the range of 1-3%, making them suitable for many analytical applications.
Stripping Methods
Stripping analysis is a highly sensitive voltammetric technique used for the determination of trace and ultratrace concentrations of metal ions (and some organic species). It involves two distinct steps, which provide a powerful preconcentration effect.
- Principle (Figure 23-30a):
- Electrodeposition (Preconcentration Step): The analyte is intentionally deposited onto a small working electrode (e.g., a mercury film electrode, a hanging mercury drop electrode, or a solid electrode like glassy carbon) from a stirred solution. This is done by applying a constant, sufficiently negative potential (for metal ions that are reduced) or a sufficiently positive potential (for species that are oxidized) for a precisely controlled period (typically several minutes). During this time, the analyte accumulates on or within the electrode material, effectively preconcentrating it from a large volume of solution into a tiny electrode volume. Stirring enhances the rate of mass transport during this step.
- Stripping Step: After the deposition step, the stirring is stopped, and the potential is scanned linearly (or by pulses, as in differential-pulse stripping voltammetry) in the reverse direction (e.g., positive-going scan for deposited metals). The deposited analyte is then “stripped” or re-oxidized (or re-reduced) back into the solution, producing a sharp current peak (Figure 23-30b).
- Advantages:
- Exceptional Sensitivity: The preconcentration step is the key to the high sensitivity, allowing detection limits ranging from 10⁻⁶ M down to 10⁻⁹ M (parts per billion to parts per trillion levels). This makes stripping methods invaluable for environmental monitoring and clinical analysis.
- Relatively Simple and Rapid: Once the method is optimized, the analysis itself is quite straightforward and can be completed in a reasonable timeframe.
- Electrodes:
- Hanging Mercury Drop Electrode (HMDE): A popular choice due to its reproducible surface and the ability of many metals to form amalgams with mercury, which facilitates their deposition and stripping.
- Mercury Film Electrodes (MFE): A thin film of mercury deposited on an inert substrate (e.g., glassy carbon). MFEs offer a larger surface area-to-volume ratio than HMDEs, leading to higher sensitivity and faster analysis.
- Solid Electrodes: Gold, silver, platinum, and carbon (especially glassy carbon) are used for analytes that do not readily form amalgams or require different potential ranges (e.g., anodic stripping voltammetry for anions).
- Quantitative Results: The height or area of the stripping peak is directly proportional to the amount of analyte deposited, and thus to its concentration in the original solution. Quantitative results depend critically on controlling experimental parameters. These are electrode size, deposition time, stirring rate during deposition, and the precise control of the stripping potential scan.
Voltammetry with Microelectrodes
Microelectrodes (or ultramicroelectrodes, UMEs) are working electrodes with extremely small characteristic dimensions, typically smaller than 20 µm, ranging down to nanometers (e.g., 30 nm in diameter). Their unique properties lead to significant advantages in voltammetry.
- Dimensions: These electrodes are microscopic, often fabricated from carbon fibers, gold, or platinum wires sealed in glass, or as arrays.
- Types:
- Planar Electrodes: Very small disks, bands, or arrays of carbon fiber, gold, or platinum wires.
- Mercury Microelectrodes: Tiny mercury drops or films electrodeposited on carbon or metal substrates.
- Advantages:
- Simpler Instrumentation (Two-Electrode System): Due to the extremely small currents generated (picoampere to nanoampere range), the IR (ohmic) drop across the solution is negligible. This often eliminates the need for a separate reference and counter electrode; a two-electrode system (working and combined reference/counter) can be used, simplifying the experimental setup.
- Reduced Capacitive Charging Currents: The charging current, which is proportional to the electrode area, is significantly reduced for microelectrodes. This leads to an improved signal-to-noise ratio and lower detection limits.
- Faster Mass Transport (Steady-State Currents): For sufficiently small microelectrodes, mass transport to the electrode surface becomes dominated by spherical (or radial) diffusion rather than planar diffusion. This leads to a very rapid establishment of steady-state currents (within microseconds), allowing for:
- The study of very rapid electrochemical reactions.
- The detection of short-lived reaction intermediates.
- Measurements in highly resistive (low dielectric constant) solvents without significant IR drop distortion.
- Applicability to Low Dielectric Constant Solvents: The minimal IR drop makes microelectrodes ideal for electrochemistry in non-aqueous and highly resistive organic solvents, which are often used for studying organic and organometallic compounds.
- Biological & Nanoscience Applications: Microelectrodes are invaluable tools for:
- In vivo measurements: Studying chemical processes in single living cells, tissues, or even individual neurons (e.g., neurotransmitter detection).
- Analysis in minuscule volumes: Performing electrochemical measurements in very small sample volumes (e.g., microdroplets, capillaries), which is crucial in nanoscience and microfluidics.
- Biosensors: Integration into highly localized and selective biosensors.
Questions and Problems (Examples from the textbook)
- Distinguish between:
- Voltammetry and Amperometry: Voltammetry measures current as a function of applied potential, while amperometry measures current at a fixed potential (often in the limiting current region).
- Linear-scan voltammetry and Pulse voltammetry: Linear-scan uses a steadily changing potential; pulse voltammetry uses discrete potential pulses with current measurements taken at specific times during the pulse cycle to reduce capacitive currents.
- Differential-pulse voltammetry and Square-wave voltammetry: Both are pulse techniques. DPV superimposes small pulses on a linear scan/staircase and measures the current difference. SWV uses a symmetrical square-wave superimposed on a staircase, measuring current differences for both forward and reverse pulses, offering much faster scans.
- Rotating disk electrode and Rotating ring-disk electrode: RDE has a single rotating disk; RRDE has an additional concentric ring electrode for detecting intermediates generated at the disk.
- Limiting current and Diffusion current: Limiting current is the maximum current achieved when the reaction rate is limited by mass transport. Diffusion current is the component of the limiting current specifically due to diffusion (often used synonymously with limiting current when convection/migration are minimized).
- Laminar flow and Turbulent flow: Laminar flow is smooth, orderly fluid motion; turbulent flow is chaotic and irregular. Laminar flow is preferred in hydrodynamic voltammetry for predictable mass transport.
- Standard electrode potential (E0) and Half-wave potential (E1/2) for a reversible reaction: E0 is a thermodynamic property of the half-reaction under standard conditions. E1/2 is the potential at which the current is half the limiting current in a voltammogram; for a reversible reaction, E1/2 is closely related to E^0′ (formal potential) and provides qualitative identification.
- Stripping methods and Standard voltammetry: Standard voltammetry directly measures the current response from species in bulk solution. Stripping methods involve a preconcentration (electrodeposition) step followed by a stripping step to dissolve the deposited analyte, significantly enhancing sensitivity.
- Define:
- Voltammograms: Plots of current versus applied potential.
- Hydrodynamic voltammetry: Voltammetry performed in a stirred or flowing solution, or with a rotating electrode, to control mass transport by convection.
- Nernst diffusion layer: A thin, stagnant layer of solution adjacent to the electrode surface where mass transport occurs solely by diffusion.
- Mercury film electrode: A thin layer of mercury deposited on an inert substrate, used as a working electrode.
- Half-wave potential (E1/2): The potential at which the current in a voltammogram is half of the limiting current.
- Diffusion current (id): The component of the limiting current resulting from the diffusion of analyte to the electrode surface.
- Why a high supporting electrolyte concentration is used: To minimize analyte migration (movement due to electric field) and to reduce the solution’s ohmic resistance (IR drop).
- Why the reference electrode is placed near the working electrode: To minimize the IR drop (voltage drop due to solution resistance) between the working electrode and the point where the reference electrode senses the potential, ensuring accurate potential control.
- Why solutions are buffered in organic voltammetry: To control the pH, as many organic electrochemical reactions involve proton transfer, and their potentials are highly pH-dependent.
- Why stripping methods are more sensitive: Because of the preconcentration step, where the analyte is accumulated onto or into the electrode before measurement, effectively increasing its localized concentration.
- Purpose of electrodeposition step in stripping analysis: To preconcentrate the analyte from a dilute solution onto a small electrode surface, thereby dramatically lowering the detection limit.
- Advantages/disadvantages of HMDE vs. other electrodes:
- HMDE Advantages: Reproducible, fresh surface with each drop; high hydrogen overvoltage allowing for reduction of many species; forms amalgams with many metals.
- HMDE Disadvantages: Mercury toxicity concerns; limited positive potential range (mercury oxidizes); cumbersome apparatus; lower surface area than mercury film electrodes.
- Solid Electrodes (e.g., glassy carbon) Advantages: Wider positive potential range; no mercury toxicity; can be easily modified.
- Solid Electrodes Disadvantages: Surface fouling issues; require careful polishing/cleaning; generally higher hydrogen overvoltage means some reductions are not possible without interference.
- Determining ‘n’ from Equation 23-13: For a reversible reaction, a plot of Eappl versus logil−ii will yield a straight line with a slope of −n0.0592 at 25°C. From this slope, n can be calculated.
- Calculations involving half-wave potentials, limiting currents, and concentrations: These problems typically involve using the Ilkovic equation (for polarography) or the direct proportionality of limiting current to concentration, along with calibration curves or standard addition methods.
Multiple Choice Questions (MCQs)
Here are 30 multiple-choice questions with answers and explanations, covering the concepts discussed in Chapter 23 on Voltammetry.
- Which statement best defines voltammetry? A) Measures potential at zero current. B) Measures charge consumed during electrolysis. C) Measures current as a function of applied potential under conditions of polarization. D) Measures resistance as a function of temperature.Answer: C Explanation: Voltammetry is defined by measuring the current response to a varying applied potential, typically with a small working electrode that becomes polarized.
- What is the primary purpose of using a small working electrode in voltammetry? A) To minimize solution resistance. B) To ensure complete concentration polarization. C) To allow for very large currents. D) To facilitate the formation of amalgams.Answer: B Explanation: A small working electrode’s limited surface area quickly leads to the analyte concentration at its surface dropping to zero, ensuring that the current is limited by the rate of mass transport (concentration polarization).
- How does voltammetry differ from coulometry in terms of analyte consumption? A) Voltammetry consumes all analyte, coulometry consumes minimal. B) Voltammetry consumes minimal analyte, coulometry consumes virtually all. C) Both consume all analyte. D) Neither consumes any analyte.Answer: B Explanation: Voltammetry is designed for minimal analyte consumption, focusing on kinetic and mechanistic information, whereas coulometry aims for complete conversion of the analyte.
- Which excitation signal produces a derivative-type curve with peaks, enhancing sensitivity? A) Linear Scan B) Triangular Waveform C) Differential Pulse D) Constant PotentialAnswer: C Explanation: Differential Pulse Voltammetry records the difference in current before and after a small pulse, resulting in a peak-shaped voltammogram which improves sensitivity and resolution.
- What is the function of the potentiostat in a voltammetric setup? A) To measure the solution’s pH. B) To maintain the working electrode potential at a controlled value relative to the reference electrode. C) To stir the solution at a constant rate. D) To remove oxygen from the solution.Answer: B Explanation: A potentiostat is an electronic device crucial for controlling and maintaining the desired potential of the working electrode relative to the reference electrode.
- Which type of working electrode is historically significant for polarography and offers a reproducible, fresh metallic surface? A) Glassy Carbon Electrode B) Platinum Disk Electrode C) Dropping Mercury Electrode (DME) D) Gold MicroelectrodeAnswer: C Explanation: The Dropping Mercury Electrode (DME) is the characteristic working electrode of classical polarography, providing a constantly renewed surface.
- What is the primary role of the supporting electrolyte in a voltammetric cell? A) To react with the analyte. B) To enhance analyte migration to the electrode. C) To minimize analyte migration and lower solution resistance. D) To serve as the reference electrode.Answer: C Explanation: A high concentration of supporting electrolyte ensures that migration of the analyte is negligible and reduces the ohmic drop in the solution.
- The constant current plateau observed at the top of a voltammetric wave, where the rate of mass transfer limits the current, is called the: A) Residual current B) Charging current C) Limiting current D) Peak currentAnswer: C Explanation: The limiting current (il) is reached when the rate of electrochemical reaction is solely determined by the rate at which the analyte can be supplied to the electrode surface.
- What does the half-wave potential (E1/2) primarily indicate for a reversible reaction? A) The maximum current achieved. B) The kinetics of the electron transfer. C) The qualitative identity of the electroactive species. D) The concentration of the supporting electrolyte.Answer: C Explanation: For reversible reactions, E1/2 is characteristic of the redox couple and is useful for qualitative identification, being closely related to the standard potential.
- In hydrodynamic voltammetry, how is mass transport to the electrode surface primarily enhanced and controlled? A) By maximizing migration. B) By using very high concentrations of analyte. C) By continuously moving the solution or the electrode. D) By reducing the temperature of the solution.Answer: C Explanation: Hydrodynamic voltammetry involves controlled motion (stirring, rotating electrode, or flowing solution) to enhance and stabilize mass transport by convection.
- The Nernst diffusion layer is a thin stagnant layer where mass transport occurs solely by: A) Migration B) Convection C) Diffusion D) EvaporationAnswer: C Explanation: Within the Nernst diffusion layer, the bulk solution flow is negligible, and therefore, mass transport to the electrode surface is governed by diffusion alone.
- Which equation relates the limiting current to the rotation speed of a rotating disk electrode (RDE)? A) Nernst Equation B) Ilkovic Equation C) Randles-Sevcik Equation D) Levich EquationAnswer: D Explanation: The Levich equation specifically describes the limiting current at a rotating disk electrode as a function of the rotation rate.
- What is a major advantage of amperometric titrations for endpoint detection? A) They do not require a working electrode. B) They are only suitable for colored solutions. C) They can be used even when the reaction product is insoluble. D) They measure pH changes at the endpoint.Answer: C Explanation: Amperometric titrations rely on current changes, and thus can effectively detect endpoints even when precipitation occurs, which might obscure optical or potentiometric detection.
- What is a key distinction of polarography compared to other forms of voltammetry? A) It always uses solid electrodes. B) It relies solely on convection for mass transport. C) It uses a dropping mercury electrode (DME) and mass transport is primarily by diffusion. D) It applies a triangular waveform.Answer: C Explanation: The use of the DME and the reliance on diffusion (with minimal convection or migration) are defining characteristics of classical polarography.
- What causes the periodic fluctuations in current observed in polarography (Figure 23-21)? A) Changes in applied potential. B) Stirring of the solution. C) Growth and dislodgment of mercury drops. D) Temperature fluctuations.Answer: C Explanation: The current changes as the surface area of the mercury drop grows and then sharply drops when a new drop forms, leading to the characteristic oscillations.
- The Ilkovic equation describes the relationship between the polarographic diffusion current and: A) The solution pH. B) The mass flow rate of mercury and drop time. C) The electrode potential. D) The reference electrode potential.Answer: B Explanation: The Ilkovic equation quantifies the diffusion current in terms of parameters related to the mercury drop, such as its flow rate (m) and drop time (t), along with the analyte’s diffusion coefficient and concentration.
- What is the main reason for the decline in importance of classical polarography? A) It’s too fast. B) Concerns about mercury toxicity and limited sensitivity compared to newer methods. C) It cannot be automated. D) It requires very small sample volumes.Answer: B Explanation: Environmental concerns with mercury and the superior sensitivity and speed of newer voltammetric techniques contributed to the decline of classical polarography.
- Which voltammetric technique primarily uses a triangular voltage waveform and is excellent for studying reaction mechanisms? A) Amperometry B) Hydrodynamic Voltammetry C) Cyclic Voltammetry (CV) D) Differential Pulse VoltammetryAnswer: C Explanation: Cyclic Voltammetry (CV) uses a triangular waveform to sweep the potential in both forward and reverse directions, providing extensive information about redox processes and intermediates.
- For a reversible electrochemical reaction in cyclic voltammetry, what is the approximate peak separation (ΔEp) at 25°C for a one-electron transfer (n=1)? A) 0 V B) 0.0592 V C) 0.1184 V D) 0.200 VAnswer: B Explanation: For a reversible reaction, ΔEp=n0.0592 V. So for n=1, ΔEp=0.0592 V.
- The Randles-Sevcik equation indicates that the peak current in cyclic voltammetry is directly proportional to: A) The square of the scan rate. B) The concentration of the supporting electrolyte. C) The square root of the scan rate and the analyte concentration. D) The solution temperature.Answer: C Explanation: The Randles-Sevcik equation states ip∝Cν1/2, meaning peak current is directly proportional to concentration and the square root of the scan rate.
- What is the main advantage of pulse voltammetry techniques (e.g., DPV, SWV) over linear-scan voltammetry? A) They are simpler to implement. B) They completely eliminate residual current. C) They significantly improve detection limits by minimizing charging current interference. D) They require larger sample volumes.Answer: C Explanation: Pulse techniques are designed to measure faradaic current when the charging current has largely decayed, leading to much lower detection limits.
- What is the typical range of detection limits for differential-pulse voltammetry? A) 10⁻¹ to 10⁻³ M B) 10⁻⁵ M C) 10⁻⁷ to 10⁻⁸ M D) 10⁻¹⁰ to 10⁻¹² MAnswer: C Explanation: Differential-pulse voltammetry offers significantly improved sensitivity, allowing for detection limits in the nanomolar range.
- Which voltammetric technique is known for its exceptional speed, capable of acquiring a full voltammogram in less than 10 milliseconds? A) Linear Scan Voltammetry B) Cyclic Voltammetry C) Differential Pulse Voltammetry D) Square-Wave VoltammetryAnswer: D Explanation: Square-wave voltammetry is characterized by its very rapid scan rates due to its unique pulse train.
- In stripping analysis, the first step, where the analyte is accumulated onto the working electrode, is called: A) Voltammetric scan B) Electrodeposition C) Amperometric measurement D) ConvectionAnswer: B Explanation: Electrodeposition is the preconcentration step where the analyte is deposited onto the electrode surface.
- What is the primary advantage of stripping methods over standard voltammetry for trace analysis? A) Simpler instrumentation. B) Higher precision. C) Significantly enhanced sensitivity due to preconcentration. D) Faster analysis time (total experiment).Answer: C Explanation: The preconcentration step in stripping analysis allows for the determination of much lower concentrations of analytes.
- Which of the following is a common working electrode used in stripping analysis for metal ions? A) Glass pH electrode B) Saturated Calomel Electrode (SCE) C) Hanging Mercury Drop Electrode (HMDE) D) Platinum wire counter electrodeAnswer: C Explanation: The Hanging Mercury Drop Electrode (HMDE) is a popular choice for stripping analysis, especially for metals that form amalgams.
- What is a key advantage of using microelectrodes in voltammetry? A) They require very large sample volumes. B) They completely eliminate all faradaic currents. C) They simplify instrumentation by minimizing IR drop and reducing capacitive currents. D) They are only suitable for non-aqueous solutions.Answer: C Explanation: The small size of microelectrodes leads to very small currents, thus negligible IR drop, and significantly reduced capacitive currents, simplifying the electrochemical cell setup.
- Mass transport to microelectrodes is primarily dominated by which type of diffusion? A) Planar diffusion B) Linear diffusion C) Spherical (or radial) diffusion D) Convective diffusionAnswer: C Explanation: Due to their small size, microelectrodes exhibit spherical or radial diffusion, which is much faster than planar diffusion and leads to rapid establishment of steady-state currents.
- Why are microelectrodes particularly useful for in vivo measurements in biological systems? A) They are very inexpensive. B) They can operate in highly conductive environments. C) Their small size allows for localized measurements in small volumes (e.g., single cells). D) They have a very high overvoltage for all reactions.Answer: C Explanation: The microscopic dimensions of UMEs allow them to be inserted into biological environments for highly localized measurements without significant perturbation.
- What is the purpose of signal averaging in voltammetric techniques like square-wave voltammetry? A) To increase the total analysis time. B) To decrease the sensitivity. C) To improve the precision and signal-to-noise ratio. D) To change the half-wave potential.Answer: C Explanation: Averaging multiple rapid scans helps to reduce random noise, thereby improving the overall precision and the signal-to-noise ratio of the measurement.