Systematic Qualitative Analysis: A Concise Guide
This document provides a concise overview of systematic qualitative analysis, a chemical process for identifying the ionic components (cations and anions) in inorganic salts. It covers essential preliminary tests, specific confirmatory tests, and critical laboratory safety measures.
1. Introduction to Qualitative Analysis
Qualitative analysis identifies the nature and identity of substances and their constituents. For inorganic salts, this means pinpointing cations (from bases) and anions (from acids). It’s performed on various scales: macro (0.1-0.5g substance, 20mL solution), semimicro (0.05g substance, 1mL solution), and micro (very small amounts).
Key observable reactions include:
- Precipitate formation
- Colour change
- Gas evolution
The process involves:
- Preliminary examination (dry tests): Initial clues from physical properties (colour, smell, solubility) and specific tests (heating, flame, borax bead, charcoal cavity).
- Anion determination: Wet tests and confirmatory tests.
- Cation determination: Wet tests and confirmatory tests.
Salt solubility and solution pH also offer important insights. Preliminary tests are always conducted before confirmatory tests.
2. Experiment: Detecting Cations and Anions
Aim: To identify one cation and one anion from a given salt, choosing from a specified list of common ions (excluding insoluble salts).
Theory: Based on:
- Solubility Product (Ksp): Precipitation occurs when the ionic product exceeds Ksp.
- Common Ion Effect: Used to control ionic product and influence precipitation.
Materials: Standard lab glassware and reagents.
3. Systematic Analysis of Anions
Step I: Preliminary Test with Dilute Sulphuric Acid
This test identifies anions (CO32−, S2−, SO32−, NO2−, CH3COO−) that evolve characteristic gases with dilute H2SO4.
Procedure: Add 1-2 mL dilute H2SO4 to ~0.1 g salt. Observe at room temperature, then warm. Test evolved gas.

Confirmatory Tests for Dilute Acid Group Anions
Tests use water extract (soluble salts) or sodium carbonate extract (insoluble salts).
Preparation of Sodium Carbonate Extract: Boil 1g salt + 3g Na2CO3 in 15mL distilled water for 10 min. Filter.
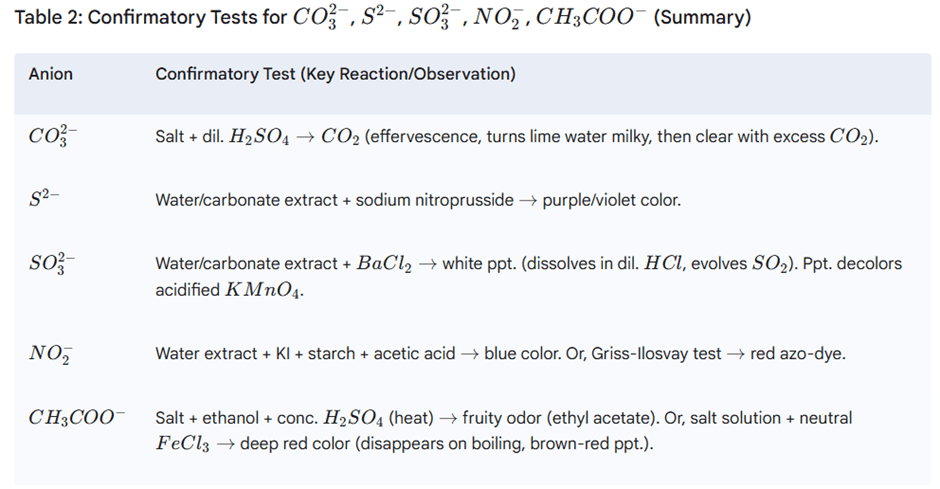
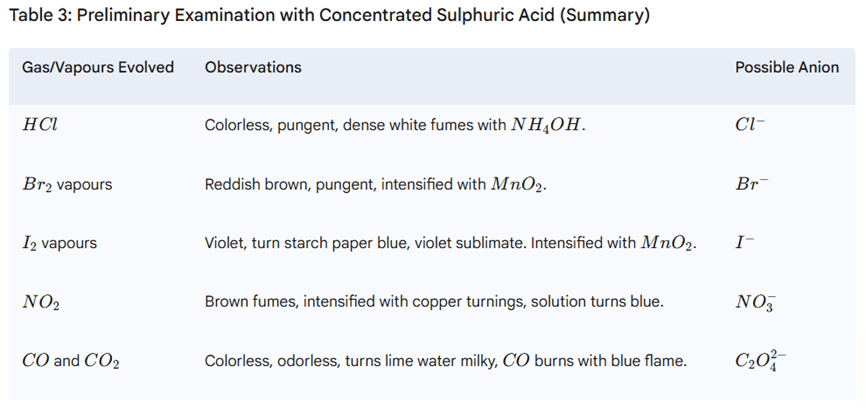
Step II: Preliminary Test with Concentrated Sulphuric Acid
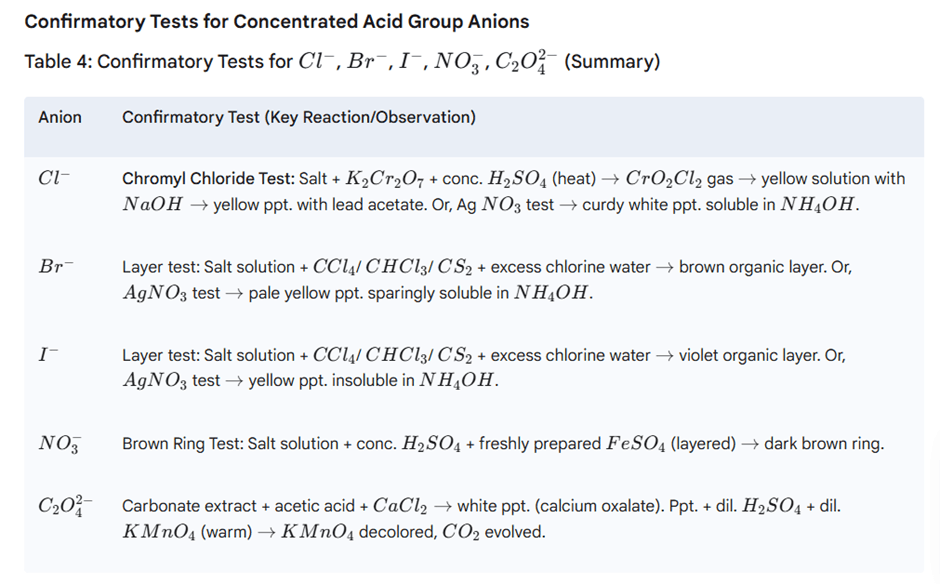
Performed if dilute H2SO4 tests are negative. Identifies Cl−, Br−, I−, NO3−, C2O42−.
Procedure: Add 3-4 drops conc. H2SO4 to ~0.1 g salt. Observe cold, then warm.
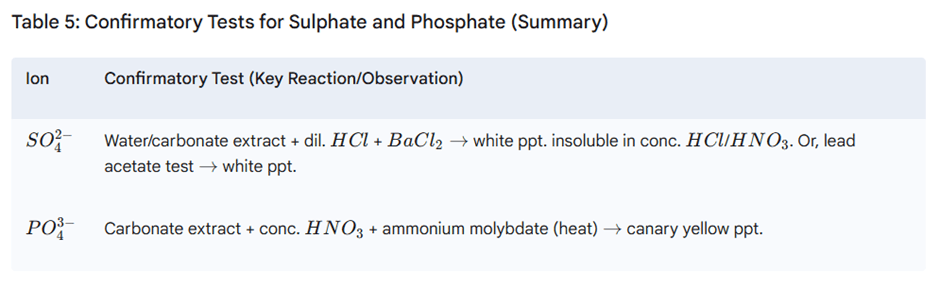
Step III: Test for Sulphate and Phosphate
Performed if previous tests are negative.
4. Systematic Analysis of Cations
Step I: Preliminary Examination of the Salt for Cation Identification
- Colour Test: Salt colour indicates possible cations (e.g., blue for Cu2+, light green for Fe2+).
- Dry Heating Test: Colour change of residue on heating/cooling (e.g., Zn2+ salts are yellow hot, white cold).
- Flame Test: Platinum wire + conc. HCl + salt paste in non-luminous flame → characteristic flame colours (e.g., green for Cu2+, crimson red for Sr2+).
- Borax Bead Test: For coloured salts. Borax bead + salt in oxidizing/reducing flame → characteristic bead colours (e.g., blue/green for Cu2+ in oxidizing flame).
- Charcoal Cavity Test: Salt + Na2CO3 in charcoal cavity (reducing flame) → coloured residue/metallic bead (e.g., yellow hot/white cold for Zn2+).
- Cobalt Nitrate Test: For white charcoal cavity residues. Residue + cobalt nitrate (heat) → characteristic colours (e.g., green for Zn2+, pink for Mg2+).
Step II: Wet Tests for Cation Identification (Group Analysis)
Preparation of Original Solution (O.S.): Dissolve salt sequentially in: distilled water → dil. HCl→ conc. HCl→ dil. HNO3→ aqua regia.
Flow Chart for Cation Separation (Summary): Cations are precipitated group-wise using specific reagents.
- Zero Group (NH4+): No group reagent. Tested first.
- Group I (Pb2+): Precipitated by dil. HCl.
- Group II (Pb2+, Cu2+, As3+): Precipitated by H2S in dil. HCl.
- Group III (Al3+, Fe3+): Precipitated by NH4OH in presence of NH4Cl.
- Group IV (Co2+, Ni2+, Mn2+, Zn2+): Precipitated by H2S in NH4OH.
- Group V (Ba2+, Sr2+, Ca2+): Precipitated by (NH4)2CO3 in NH4OH.
- Group VI (Mg2+): No group reagent. Tested last.
(I) Zero Group Cation (NH4+)
Test: Salt + NaOH (heat) → ammonia gas (smell, white fumes with HCl). Confirmed by brown ppt. with Nessler’s reagent.
(II) Group-I Cation (Pb2+)
Test: White ppt. with dil. HCl. Confirmed by:
- Yellow ppt. with KI (soluble in hot water, shining crystals on cooling).
- Yellow ppt. with K2CrO4 (soluble in NaOH).
- White ppt. with dil. H2SO4 (soluble in ammonium acetate).
(III) Group–II Cations (Pb2+, Cu2+, As3+)
Test: Precipitate with H2S in dil. HCl. Black (Pb2+, Cu2+), yellow (As3+).
- Group II-A (Copper Group – Insoluble in yellow ammonium sulphide):
- Pb2+: White ppt. (PbSO4) after dissolving sulphide in HNO3 and adding H2SO4. Confirmed by yellow ppts. with K2CrO4 and KI.
- Cu2+: Blue solution with excess NH4OH. Confirmed by chocolate brown ppt. with K4[Fe(CN)6].
- Group II-B (Arsenic Group – Soluble in yellow ammonium sulphide):
- As3+: Yellow ppt. (As2S5) after acidification. Confirmed by canary yellow ppt. with ammonium molybdate.
(IV) Group–III Cations (Fe3+, Al3+)
Test: Precipitate with NH4Cl + excess NH4OH. Brown (Fe3+), gelatinous white (Al3+).
- Al3+: White gelatinous ppt. with NaOH (soluble in excess NaOH). Confirmed by blue lake test.
- Fe3+: Confirmed by blue ppt. with K4[Fe(CN)6] (Prussian blue) or blood red colour with KSCN.
(V) Group–IV Cations (Co2+, Ni2+, Mn2+, Zn2+)
Test: Precipitate with H2S. White (Zn2+), flesh (Mn2+), black (Ni2+, Co2+).
- Zn2+: White ppt. with NaOH (soluble in excess NaOH). Confirmed by bluish white ppt. with K4[Fe(CN)6].
- Mn2+: White ppt. with NaOH (turns brown on standing).
- Ni2+: Black sulphide. Confirmed by bright red ppt. with dimethylglyoxime.
- Co2+: Black sulphide. Confirmed by yellow ppt. with potassium nitrite (potassium hexanitritocobaltate(III)).
(VI) Group–V Cations (Ba2+, Sr2+, Ca2+)
Test: White ppt. with (NH4)2CO3 + NH4OH. Dissolve ppt. in acetic acid.
- Ba2+: Yellow ppt. with K2CrO4. Flame test: grassy green.
- Sr2+: White ppt. with (NH4)2SO4. Flame test: crimson-red.
- Ca2+: White ppt. with ammonium oxalate. Flame test: brick red (greenish-yellow through blue glass).
(VII) Group–VI Cation (Mg2+)
Test: If Group-V is absent. Original solution + NH4OH + Na2HPO4 (heat, scratch) → white crystalline ppt. (Mg(NH4)PO4).
5. Result
Example: The given salt contains Anion: SO42− and Cation: Mg2+.
6. Precautions
Always prioritize safety:
- Wear PPE (apron, eye protector, gloves).
- Read labels, never taste chemicals.
- Fan vapours, don’t inhale directly.
- Never add water to acid.
- Point test tubes away from people when heating.
- Handle explosives, flammables, poisons, electricity, and hot items with extreme care.
- Maintain a clean workspace, use dustbins.
- Wash hands after lab work.
- Use minimum reagent quantities.
Pingback: Class 11 Chemistry Half Yearly Exam: Important | Studychem