Molecular Analysis of Organic Compounds
Molecular analysis of organic compounds involves determining their elemental composition, molecular mass, and ultimately, their empirical and molecular formulas. This process is crucial for characterizing unknown organic substances.
1. General Principles
The molecular analysis typically involves two main stages:
- Qualitative Analysis: Detection of elements present in the organic compound (Carbon, Hydrogen, Oxygen, Nitrogen, Sulphur, Halogens, Phosphorus, etc.).
- Quantitative Analysis: Estimation of the percentage composition of each element present.
- Determination of Molecular Mass: Using various methods.
- Calculation of Empirical and Molecular Formulas: Combining the above data.
2. Detection of Elements (Qualitative Analysis)
Organic compounds typically contain carbon and hydrogen. Other elements like oxygen, nitrogen, sulphur, and halogens may also be present.
2.1. Detection of Carbon and Hydrogen
- Principle: When an organic compound is heated with dry copper (II) oxide (CuO), carbon is oxidized to carbon dioxide (CO2) and hydrogen to water (H2O).
- Procedure:
- Organic compound + dry CuO (black) are heated in a combustion tube.
- Gases passed through anhydrous copper sulphate (CuSO4) (white).
- Then passed through lime water Ca(OH)2.
- Observations:
- Hydrogen: Anhydrous CuSO4 turns blue (confirms H2O formation).
- H + CuO → H2O + Cu
- CuSO4 (white) + 5H2O → CuSO4⋅5H2O (blue)
- Carbon: Lime water turns milky (confirms CO2 formation).
- C + CuO → CO2 + Cu
- CO2 +Ca(OH)2 → CaCO3↓ (white ppt) + H2O
- Hydrogen: Anhydrous CuSO4 turns blue (confirms H2O formation).
2.2. Detection of Other Elements (Lassaigne’s Test)
Nitrogen, Sulphur, and Halogens are detected using Lassaigne’s test (Sodium Fusion Test). This test converts these elements into water-soluble ionic compounds by fusing the organic compound with metallic sodium. This is because organic compounds are covalent, and direct detection of these elements in their covalent forms is difficult.
- Principle: Fusing an organic compound with sodium metal converts elements like N, S, and X (halogens) into sodium cyanide (NaCN), sodium sulfide (Na2S), and sodium halide (NaX) respectively, which are ionic and water-soluble. The fused mass is then extracted with distilled water to prepare Lassaigne’s extract (sodium fusion extract).
- Procedure:
- Fusion: Small piece of sodium metal is heated strongly with the organic compound in a fusion tube until red hot.
- Quenching: The red-hot tube is plunged into distilled water in a porcelain dish.
- Boiling: The contents are boiled, crushed, and filtered to get Lassaigne’s extract.
- Tests on Lassaigne’s Extract:
- Detection of Nitrogen:
- Test: To a portion of Lassaigne’s extract, add freshly prepared ferrous sulfate solution (FeSO4), boil, cool, and acidify with concentrated sulfuric acid (H2SO4).
- Observation: Formation of a Prussian blue color or precipitate indicates the presence of nitrogen.
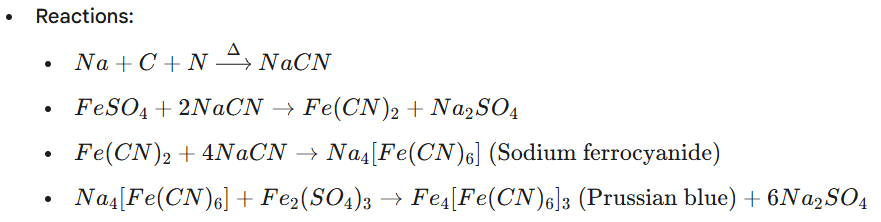
- Detection of Sulfur:
- Test 1 (Sodium Nitroprusside Test): To a portion of Lassaigne’s extract, add a few drops of sodium nitroprusside solution.
- Observation: Violet or purple color indicates sulphur.
- Na2S + Na2[Fe(CN)5NO] → Na4[Fe(CN)5NOS] (Violet color)
- Test 2 (Lead Acetate Test): To a portion of Lassaigne’s extract, acidify with acetic acid, then add lead acetate solution.
- Observation: Black precipitate of lead sulfide (PbS) indicates sulfur.
- Na2S + (CH3COO)2Pb → PbS↓ (black ppt) + 2CH3COONa
- Note: If both N and S are present, NaCNS (sodium thiocyanate) is formed, which gives blood-red color with FeCl3.
- Detection of Halogens (Cl, Br, I):
- Test: Acidify a portion of Lassaigne’s extract with dilute HNO3 (boil to expel HCN and H2S if N and S are present, otherwise they interfere) and then add silver nitrate solution (AgNO3).
- Observations:
- Chlorine: White precipitate of AgCl soluble in ammonium hydroxide (NH4OH).
- Bromine: Pale yellow precipitate of AgBr sparingly soluble in NH4OH.
- Iodine: Yellow precipitate of AgI insoluble in NH4OH.
- Reactions: NaX + AgNO3 → AgX↓ + NaNO3
2.3. Detection of Oxygen
- Oxygen cannot be detected by Lassaigne’s test. Its presence is usually inferred from the molecular formula after quantitative analysis of other elements, or by specific methods like the direct method (heating with graphite).
2.4. Detection of Phosphorus
- Test: The organic compound is heated with an oxidizing agent (Na2O2 or conc. HNO3) to oxidize phosphorus to phosphate. The solution is then treated with ammonium molybdate.
- Observation: Yellow precipitate of ammonium phosphomolybdate indicates phosphorus.
3. Estimation of Elements (Quantitative Analysis)
This involves determining the precise percentage of each element.
3.1. Estimation of Carbon and Hydrogen (Liebig’s Method)
- Principle: A known mass of the organic compound is completely burnt in a current of dry oxygen in the presence of copper (II) oxide. Carbon is oxidized to CO2 and hydrogen to H2O. The masses of CO2 and H2O produced are measured.
- Procedure:
- Sample burnt in combustion tube.
- H2O absorbed by anhydrous CaCl2 in a U-tube.
- CO2 absorbed by KOH solution in a U-tube.
- Calculations:
- The percentage of Hydrogen is calculated by dividing the mass of water formed by the mass of the organic compound, multiplying by the ratio of the molar mass of 2 Hydrogens to Water (2/18), and then by 100.
- The percentage of Carbon is calculated by dividing the mass of Carbon Dioxide formed by the mass of the organic compound, multiplying by the ratio of the molar mass of Carbon-to-Carbon Dioxide (12/44), and then by 100.
3.2. Estimation of Nitrogen
- Dumas Method (for Nitrogen):
- Principle: A known mass of organic compound is heated with copper (II) oxide in an atmosphere of CO2. Nitrogen (from the compound) is converted to free nitrogen gas (N2). Other elements are oxidized to CO2 and H2O. The volume of N2 gas collected is measured.
- Procedure: Sample combusted, gases passed over heated copper gauze (to reduce nitrogen oxides), then collected over KOH solution (absorbs CO2) in a nitrometer.
- Calculation: Using ideal gas law, volume of N2 at STP is calculated, then its mass, and finally percentage.
- The percentage of Nitrogen is calculated by taking the molar mass of N2 (28) divided by its molar volume at STP (22400 mL), multiplying by the volume of N2 at STP divided by the mass of the organic compound, and then by 100.
- Kjeldahl’s Method (for Nitrogen):
- Principle: A known mass of organic compound containing nitrogen is heated with concentrated H2SO4 (digestion). Nitrogen is quantitatively converted to ammonium sulphate ((NH4)2SO4). This is then heated with excess NaOH to liberate ammonia (NH3), which is absorbed in a known volume of standard acid (H2SO4). The unreacted acid is back-titrated.
- Limitation: Not suitable for nitro groups, azo groups, or nitrogen in ring compounds (e.g., pyridine) as nitrogen is not converted to ammonium sulfate quantitatively.
- Calculation: From the amount of NH3 formed (determined by titration), the percentage of nitrogen is calculated.
- The percentage of Nitrogen is calculated using a constant (1.4 for mL and Normality), multiplied by the normality of the acid used and the volume of acid consumed by ammonia, all divided by the mass of the organic compound.
3.3. Estimation of Halogens (Carius Method)
- Principle: A known mass of organic compound is heated strongly with fuming nitric acid (HNO3) and silver nitrate (AgNO3) in a sealed Carius tube. Halogen is converted to silver halide (AgX).
- Procedure: After heating, the AgX is filtered, washed, dried, and weighed.
- Calculations:
- The percentage of Chlorine is calculated by dividing the mass of Silver Chloride formed by the mass of the organic compound, multiplying by the ratio of the atomic mass of Chlorine to the molar mass of Silver Chloride (35.5/143.5), and then by 100.
- The percentage of Bromine is calculated by dividing the mass of Silver Bromide formed by the mass of the organic compound, multiplying by the ratio of the atomic mass of Bromine to the molar mass of Silver Bromide (80/188), and then by 100.
- The percentage of Iodine is calculated by dividing the mass of Silver Iodide formed by the mass of the organic compound, multiplying by the ratio of the atomic mass of Iodine to the molar mass of Silver Iodide (127/235), and then by 100.
3.4. Estimation of Sulphur (Carius Method)
- Principle: Similar to halogen estimation, the organic compound is heated with fuming HNO3 in a Carius tube. Sulphur is oxidized to sulfuric acid (H2SO4). H2SO4 is then precipitated as barium sulphate (BaSO4) by adding BaCl2 solution.
- Procedure: BaSO4 is filtered, washed, dried, and weighed.
- Calculations:
- The percentage of Sulphur is calculated by dividing the mass of Barium Sulphate formed by the mass of the organic compound, multiplying by the ratio of the atomic mass of Sulphur to the molar mass of Barium Sulphate (32/233), and then by 100.
3.5. Estimation of Phosphorus
- Principle: The organic compound is heated with fuming HNO3. Phosphorus is oxidized to phosphoric acid (H3PO4). H3PO4 is then precipitated either as ammonium phosphomolybdate or as magnesium ammonium phosphate (MgNH4PO4), which on ignition gives magnesium pyrophosphate (Mg2P2O7).
- Calculations:
- From Mg2P2O7: The percentage of Phosphorus is calculated by dividing the mass of Magnesium Pyrophosphate formed by the mass of the organic compound, multiplying by the ratio of two times the atomic mass of Phosphorus to the molar mass of Magnesium Pyrophosphate (2×31/222), and then by 100.
3.6. Estimation of Oxygen
- Oxygen content is usually determined by difference (100%−sum of other elements’ percentages).
- Direct Method (rarely used): A known mass is decomposed in a stream of nitrogen. Oxygen is converted to CO2 by passing over red hot carbon, and the CO2 is absorbed and weighed.
4. Determination of Molecular Mass
Several methods are used, depending on the nature of the compound.
4.1. Victor Meyer’s Method (for Volatile Liquids/Solids)
- Principle: A known mass of the volatile organic compound is vaporized, and the volume of vapor displaced (air) at a known temperature and pressure is measured. This volume is equal to the volume of the organic vapor.
- Calculation: Using the ideal gas equation (PV=nRT or PV=(w/M)RT), the molecular mass (M) can be calculated.
- M=wRT/PV.
4.2. Colligative Property Methods (Cryoscopic, Ebullioscopic)
- Principle: Based on the depression of freezing point (cryoscopic) or elevation of boiling point (ebullioscopic) of a solvent by a known mass of solute.
- Calculation: Using formulas involving molal depression/elevation constant (Kf/Kb) and change in temperature (ΔTf/ΔTb).
- ΔTf = Kf × m = Kf× (w2×1000)/ (M2×w1)
- ΔTb = Kb × m = Kb× (w2×1000)/ (M2×w1)
4.3. Mass Spectrometry (Modern Method)
- Principle: Molecules are ionized, fragmented, and the mass-to-charge ratio (m/z) of the ions is measured. The molecular ion peak gives the molecular mass.
- Advantages: Highly accurate, requires very small sample size, provides structural information from fragmentation patterns.
5. Empirical Formula and Molecular Formula
- Empirical Formula: The simplest whole-number ratio of atoms present in a compound.
- Molecular Formula: The actual number of atoms of each element present in a molecule of the compound.
5.1. Steps for Calculation
- Percentage Composition: Determine the percentage composition of each element (from quantitative analysis). If oxygen is by difference, calculate it as 100−(%C + %H + %N + %S + %X).
- Relative Number of Atoms: Divide the percentage of each element by its atomic mass.
- Simple Ratio: Divide each value from step 2 by the smallest value obtained.
- Whole Number Ratio: If values from step 3 are not whole numbers, multiply by a suitable integer to get the simplest whole-number ratio. This gives the empirical formula.
- Empirical Formula Mass: Calculate the mass of the empirical formula.
- Molecular Formula Calculation:
- n= (Molecular Mass)/ (Empirical Formula Mass)
- Molecular Formula = n × Empirical Formula.
- Molecular mass can be determined by methods like Victor Meyer’s or mass spectrometry.
This comprehensive analysis allows chemists to fully characterize and understand the composition of organic compounds.
Pingback: General Organic Chemistry: Fundamentals | Studychem