In the current experiment, potassium permanganate (KMnO4) acts as a powerful oxidising agent. Though KMnO4 acts as an oxidising agent in alkaline medium as well, for quantitative analysis mostly acidic medium is used. The oxidising action of KMnO4 in the acidic medium can be represented by the following
equation:
MnO4– + 8H+ + 5e– → Mn2+ + 4H2O
[The acid used in this titration is dilute H2SO4. HNO3 is not used as it is itself an oxidising agent and HCl is usually avoided because it reacts with KMnO4 according to the equation given below to produce chlorine and chlorine which is also an oxidising agent in the aqueous solution.
2KMnO4 + 16HCl → 2KCl + 2MnCl2 + 5Cl2 + 8H2O]
Since, oxalic acid acts as a reducing agent, it can be titrated against potassium permanganate in the acidic medium according to the following chemical equation:
- Chemical Equation
Reduction half reaction: 2KMnO4 + 3H2SO4 → K2SO4 + 2MnSO4 + 3H2O + 5 [O]
Oxidation half reaction: H2C2O4 + [O] →2CO2 + H2O] × 5
_____________________________________________________________________
2KMnO4 + 3H2SO4 + 5H2C2O4 → K2SO4 + 2MnSO4 + 8H2O + 10CO2
- Ionic Equation
Reduction half reaction: MnO4– + 5e– + 8H+ → Mn2+ + 4H2O] × 2
Oxidation half reaction: C2O4– → 2CO2 + 2e–] × 5
2MnO4– + 5C2O42– + 16H+ → 2Mn2+ + 10CO2 + 8H2O
In the above equations, C2O42– is oxidised to CO2 and MnO4– is reduced to Mn2+. The oxidation state of carbon in C2O42– changes from +3 to +4. In the following titrations, KMnO4 acts as a self-indicator. Primarily colour of KMnO4 is discharged due to its reduction by oxalic acid. After complete consumption of oxalate ions, the end point is indicated by the presence of a light pink colour produced by the addition of a slight excess of unreacted KMnO4. Further, during the titration of oxalic acid against potassium permanganate, heating of oxalic acid solution (50°–60°C) along with dilute H2SO4 is required. This is important because the reaction takes place at higher temperature. During the titration, first manganous sulphate is formed which acts as a catalyst for the reduction of KMnO4 by oxalic acid. Therefore, in the beginning the reaction rate is slow and as the reaction proceeds, the rate of the reaction increases.
Indicator Use: KMnO4 is a self-indicator
End Point Colour: Colourless to light pink
Observation:
Weight of watch glass = 14.4738 g
Weight of watch glass + oxalic acid = 17.6238 g
Weight of oxalic acid = 3.15 g
Volume of solution prepared = 250 mL
Molarity of oxalic acid solution = 0.1 M
Volume of oxalic acid taken for each titration = 20 mL
| Sl. No. | Volume of Oxalic Acid (ml) | Burette Reading (ml) | Volume (V) of KMnO4 used V = (V2–V1) ml | Mean Volume of KMnO4 (ml) | |
| Initial (V1) | Final (V2) | ||||
| 1 | 20 | 0 | 18.5 | 18.5 | 18.5 |
| 2 | 20 | 0 | 18.5 | 18.5 | |
| 3 | 20 | 0 | 18.5 | 18.5 | |
Calculation:
Molarity of KMnO₄ solution:
From the balanced equation,
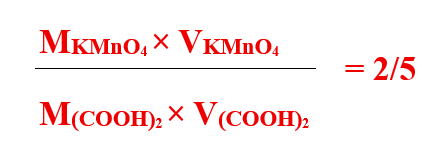
⇒MKMnO4×18.5 = 2/5 × (0.1×20)
⇒M KMnO4 = {2/5 × (0.1 × 20)}/ 18.5
⇒MKMnO4 = 0.043 M (approximately)
Strength of KMnO₄ in gL-1: = MKMnO4 × molar mass of KMnO₄ = 0.043 x 158 = 6.79 gL-1
Result: Hence the obtained strength of the given KMnO₄ solution is 0.043 M and 6.79 gL-1.
[Note:]
PROCEDURE:
- Take a clean pipette, rinse it with 0.1 M oxalic acid solution and then pipette out 20 mL of this solution into a conical flask.
- Add about one test‑tube full of dilute H₂SO₄ to the conical flask to provide an acidic medium.
- Take a clean burette, rinse and fill it with the supplied KMnO₄ solution. Place the conical flask just below the burette nozzle and adjust the height so that the nozzle tip just enters the mouth of the flask.
- Note the initial burette reading and start the titration, slowly running KMnO₄ into the heated oxalic acid solution (around 60–70°C) with constant stirring until a faint permanent pink colour persists for about 30 seconds, then note the final burette reading.
- Repeat the experiment at least thrice.