Classification and Nomenclature of Organic Compounds
Organic chemistry is the study of carbon compounds. Due to the unique properties of carbon (catenation, tetravalency), it forms an immense number of compounds, necessitating systematic classification and nomenclature.
1. Classification of Organic Compounds
Organic compounds can be broadly classified based on their carbon skeleton and the presence of functional groups.
1.1. Based on Carbon Skeleton
- Acyclic or Open-Chain Compounds (Aliphatic Compounds):
- Contain an open chain of carbon atoms, which can be straight or branched.
- Do not contain any ring structures.
- Examples: Ethane (CH3−CH3), Butane (CH3−CH2−CH2−CH3), Isobutane (CH3−CH(CH3)−CH3), Ethanol (CH3CH2OH), Acetone (CH3COCH3).
- Cyclic or Closed-Chain Compounds (Ring Compounds):
- Contain one or more rings of carbon atoms.
- Further classified into:
- Homocyclic (Carbocyclic) Compounds: Rings are composed only of carbon atoms.
- Alicyclic Compounds: Resemble aliphatic compounds in properties but contain a ring structure. They may be saturated (e.g., Cyclopropane, Cyclohexane) or unsaturated (e.g., Cyclopropene, Cyclohexene) but are not aromatic.
- Examples: Cyclopropane, Cyclohexane, Cyclohexene.
- Aromatic Compounds: Contain special stability due to delocalized π-electron systems (e.g., benzene and its derivatives). They generally follow Huckel’s Rule (4n+2 π-electrons).
- Examples: Benzene, Toluene, Naphthalene, Phenol, Aniline.
- Alicyclic Compounds: Resemble aliphatic compounds in properties but contain a ring structure. They may be saturated (e.g., Cyclopropane, Cyclohexane) or unsaturated (e.g., Cyclopropene, Cyclohexene) but are not aromatic.
- Heterocyclic Compounds: Rings contain at least one atom other than carbon (heteroatom) as part of the ring structure. Common heteroatoms include O, N, S.
- Alicyclic Heterocyclic: Non-aromatic heterocyclic rings.
- Examples: Tetrahydrofuran (THF), Tetrahydropyran, Pyrrolidine, Piperidine.
- Aromatic Heterocyclic: Heterocyclic rings that exhibit aromaticity.
- Examples: Pyridine, Pyrrole, Furan, Thiophene.
- Alicyclic Heterocyclic: Non-aromatic heterocyclic rings.
- Homocyclic (Carbocyclic) Compounds: Rings are composed only of carbon atoms.
1.2. Based on Functional Groups
Organic compounds are also classified based on the presence of functional groups. A functional group is an atom or group of atoms within a molecule that is largely responsible for the characteristic chemical reactions of that molecule. The rest of the molecule is often referred to as the alkyl (R) or aryl (Ar) group.
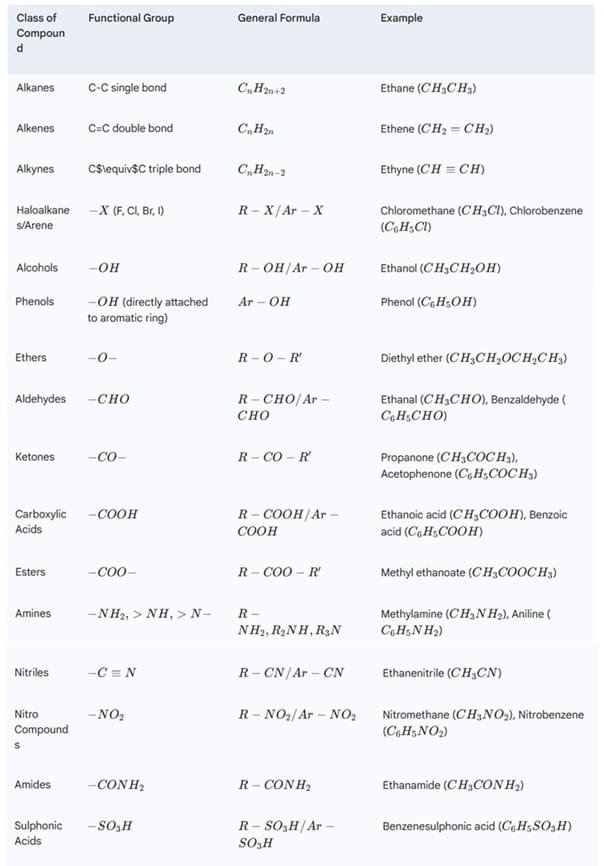
2. Nomenclature of Organic Compounds
The systematic naming of organic compounds is governed by the rules of the International Union of Pure and Applied Chemistry (IUPAC). Common names are also frequently used, especially for simpler or historically important compounds.
2.1. IUPAC Nomenclature (General Rules)
- Longest Chain Rule (Parent Chain): Identify the longest continuous carbon chain, which forms the parent hydrocarbon name. If a functional group is present, the longest chain containing the functional group should be chosen.
- Numbering the Chain:
- If a functional group is present, number the chain to give the functional group the lowest possible number.
- If multiple bonds are present, they get preference over substituents in numbering.
- If functional groups and multiple bonds are present, the functional group dictates numbering, then multiple bonds, then substituents.
- If substituents are present, number the chain to give the lowest possible sum of locants (positions). If still a tie, alphabetize.
- Naming Substituents: Alkyl groups (e.g., methyl, ethyl, propyl), halogens (fluoro, chloro, bromo, iodo), nitro, amino, etc., are named as prefixes.
- Prefixes for Number of Groups: Di-, tri-, tetra- are used for identical substituents. Bis-, tris-, tetrakis- are used for complex identical substituents (e.g., ‘bis(chloromethyl)’).
- Alphabetical Order: Substituents are listed alphabetically (ignoring di, tri, etc., except for complex ones like isopropyl or tert-butyl).
- Punctuation: Commas separate numbers, hyphens separate numbers and words. No spaces in the name.
2.2. Naming Specific Classes of Compounds
- Alkanes: Suffix “-ane”. (e.g., Methane, Ethane, Propane, Butane, etc.)
- Alkenes: Suffix “-ene”. Indicate position of double bond. (e.g., Ethene, Propene, But-1-ene, But-2-ene)
- Alkynes: Suffix “-yne”. Indicate position of triple bond. (e.g., Ethyne, Propyne, But-1-yne)
- Haloalkanes: Prefix “halo-“. (e.g., Chloromethane, 2-Bromopropane)
- Alcohols: Suffix “-ol”. Indicate position of -OH. (e.g., Ethanol, Propan-1-ol, Propan-2-ol)
- Ethers: Alkyl groups named alphabetically followed by “ether” (common) or smaller alkyl group as “alkoxy” prefix to the larger alkane. (e.g., Diethyl ether, Methoxyethane)
- Aldehydes: Suffix “-al”. Carbonyl carbon is C-1. (e.g., Ethanal, Propanal)
- Ketones: Suffix “-one”. Indicate position of carbonyl. (e.g., Propanone, Butan-2-one)
- Carboxylic Acids: Suffix “-oic acid”. Carbonyl carbon is C-1. (e.g., Ethanoic acid, Propanoic acid)
- Esters: Alkyl group of the alcohol part comes first, then name of the carboxylic acid part with “-oate” suffix. (e.g., Methyl ethanoate)
- Amines: Suffix “-amine”. Or prefix “amino-“. For 2∘/3∘ amines, N-alkyl/aryl prefixes. (e.g., Ethanamine, N-Methylethanamine, Benzenamine (Aniline))
- Aromatic Compounds: Derivatives of benzene. Use common names (Toluene, Phenol, Aniline) or systematic names (Methylbenzene, Hydroxybenzene, Aminobenzene). For disubstituted, use ortho (1,2), meta (1,3), para (1,4) or numbers.
2.3. Common Names
Many compounds retain widely accepted common names, especially for simpler structures or those frequently encountered.
- Example: Toluene (for methylbenzene), Acetone (for propanone), Chloroform (for trichloromethane), Aniline (for benzenamine).
3. Homologous Series
A homologous series is a series of organic compounds with the same general formula, similar chemical properties, and a gradual change in physical properties as the series progresses (each successive member differs by a −CH2− group).
- Characteristics:
- Same general formula (e.g., alkanes CnH2n+2).
- Successive members differ by −CH2− unit.
- Similar chemical properties due to the same functional group.
- Gradual change in physical properties (boiling point, melting point, density) with increasing molar mass.
- Can be prepared by general methods.
Understanding these classification and nomenclature rules is foundational for studying and communicating effectively in organic chemistry.
40 Important MCQs on Classification and Nomenclature of Organic Compounds with Explanations
1. Which of the following best defines an organic compound? (a) Any compound containing carbon. (b) A compound containing carbon bonded to hydrogen. (c) A compound containing carbon, hydrogen, and oxygen. (d) A compound derived from living organisms.
Explanation: The correct answer is (b). While definition (a) is often a simplification, the core definition of an organic compound is one that contains carbon atoms usually bonded to hydrogen atoms, forming the basis of carbon-hydrogen frameworks. Exceptions like CO2, carbonates, and carbides are generally considered inorganic.
2. Acyclic compounds are also known as: (a) Cyclic compounds (b) Aliphatic compounds (c) Aromatic compounds (d) Heterocyclic compounds
Explanation: The correct answer is (b). Acyclic or open-chain compounds are commonly referred to as aliphatic compounds. They do not contain any ring structures.
3. Which of the following is an example of an alicyclic compound? (a) Benzene (b) Toluene (c) Cyclohexane (d) Pyridine
Explanation: The correct answer is (c). Alicyclic compounds are cyclic aliphatic compounds that do not possess aromatic character. Cyclohexane is a saturated cyclic hydrocarbon. Benzene and Toluene are aromatic, and Pyridine is an aromatic heterocyclic compound.
4. A compound containing a ring of carbon atoms and at least one heteroatom within the ring is classified as: (a) Homocyclic (b) Alicyclic (c) Heterocyclic (d) Aromatic
Explanation: The correct answer is (c). Heterocyclic compounds are cyclic compounds where the ring contains one or more atoms other than carbon (heteroatoms) as part of the ring structure.
5. Which of the following is an aromatic heterocyclic compound? (a) Cyclohexanol (b) Tetrahydrofuran (c) Pyrrole (d) Cyclopentane
Explanation: The correct answer is (c). Pyrrole is a five-membered ring containing nitrogen as a heteroatom and exhibits aromaticity (6 π-electrons, fulfilling Huckel’s rule). Tetrahydrofuran is an alicyclic heterocyclic compound.
6. The functional group present in alcohols is: (a) −CHO (b) −COOH (c) −OH (d) −CO−Error! Filename not specified.
Explanation: The correct answer is (c). The hydroxyl group (−OH) is the characteristic functional group of alcohols.
7. Which functional group is found in ketones? (a) Carbonyl group at the end of the chain (b) Carbonyl group within the chain (c) Hydroxyl group (d) Carboxyl group
Explanation: The correct answer is (b). Ketones are characterized by a carbonyl group (−CO−) located within the carbon chain, bonded to two alkyl or aryl groups. Aldehydes have a terminal carbonyl group.
8. The general formula for alkenes is: (a) CnH2n+2 (b) CnH2n (c) CnH2n−2 (d) CnH2n−6
Explanation: The correct answer is (b). Alkenes are unsaturated hydrocarbons containing one carbon-carbon double bond, and their general formula is CnH2n.
9. What is the IUPAC name for CH3CH2CH2OH?
(a) Propanol (b) Propan-1-ol (c) n-Propyl alcohol (d) Propan-2-ol
Explanation: The correct answer is (b). According to IUPAC rules, the position of the functional group must be indicated. The hydroxyl group is on the first carbon, so it’s Propan-1-ol. “Propanol” is less specific, and “n-Propyl alcohol” is a common name. Propan-2-ol has the -OH group on the second carbon.
10. The IUPAC name for CH3COCH3 is: (a) Propanal (b) Acetone (c) Propanone (d) Propyne
Explanation: The correct answer is (c). CH3COCH3 is a ketone with three carbon atoms. The IUPAC suffix for ketones is “-one”. Acetone is its common name. Propanal is an aldehyde.
11. Which rule is used to identify the longest continuous carbon chain in IUPAC nomenclature? (a) Lowest sum rule (b) Alphabetical rule (c) Longest chain rule (d) First point of difference rule
Explanation: The correct answer is (c). The first step in IUPAC nomenclature is to identify the longest continuous carbon chain, which serves as the parent hydrocarbon.
12. In IUPAC nomenclature, if a compound has both a double bond and a hydroxyl group, which functional group gets preference in numbering? (a) Double bond (b) Hydroxyl group (c) Both equally (d) The one nearer to the end of the chain
Explanation: The correct answer is (b). Functional groups (like -OH) generally take precedence over multiple bonds (double or triple bonds) in numbering the carbon chain to assign the lowest possible locant.
13. What is the IUPAC name for CH3CH(CH3)CH2CH3? (a) n-Pentane (b) Isopentane (c) 2-Methylbutane (d) Neopentane
Explanation: The correct answer is (c). The longest chain has four carbons (butane). There is a methyl group on the second carbon. So, 2-Methylbutane. Isopentane is a common name for this compound.
14. A homologous series is characterized by: (a) Same molecular formula (b) Different chemical properties (c) Successive members differing by a −CH2− unit (d) All members being gases at room temperature
Explanation: The correct answer is (c). A defining characteristic of a homologous series is that each successive member differs from the previous one by a methylene (−CH2−) group.
15. Which of the following functional groups is a carboxyl group? (a) −CHO (b) −CO− (c) −COOH (d) −COO−
Explanation: The correct answer is (c). The carboxyl group (−COOH) is characteristic of carboxylic acids.
16. The common name for methylbenzene is: (a) Aniline (b) Phenol (c) Toluene (d) Benzaldehyde
Explanation: The correct answer is (c). Toluene is the common name for methylbenzene.
17. What is the IUPAC name for CH3COOCH2CH3? (a) Methyl ethanoate (b) Ethyl methanoate (c) Ethyl ethanoate (d) Propyl ethanoate
Explanation: The correct answer is (c). This is an ester. The alkyl group from the alcohol part (−OCH2CH3) is “ethyl”. The acid part (CH3COO−) comes from ethanoic acid. So, it’s Ethyl ethanoate.
18. Which of the following is an example of an aromatic compound? (a) Cyclobutane (b) Pyridine (c) Cyclohexene (d) Pyrrolidine
Explanation: The correct answer is (b). Pyridine is an aromatic heterocyclic compound. Cyclobutane and Cyclohexene are alicyclic. Pyrrolidine is an alicyclic heterocyclic compound.
19. The number of carbon atoms in the parent chain of 2,2,4-trimethylpentane is: (a) 5 (b) 6 (c) 7 (d) 8
Explanation: The correct answer is (a). The parent name is “pentane,” which indicates a five-carbon main chain. “Trimethyl” indicates three methyl substituents.
20. Which class of compounds has the functional group −C≡N? (a) Amides (b) Amines (c) Nitriles (d) Nitro compounds
Explanation: The correct answer is (c). The functional group −C≡N is characteristic of nitriles (or cyanides).
21. What is the IUPAC name for CH3CH2NH2? (a) Methylamine (b) Ethanamine (c) Aniline (d) Propylamine
Explanation: The correct answer is (b). This is a primary amine with two carbon atoms. The suffix “-amine” is added to the alkane name. So, Ethanamine.
22. Which of the following is a tertiary amine? (a) Methylamine (b) Dimethylamine (c) Triethylamine (d) Aniline
Explanation: The correct answer is (c). Triethylamine ((C2H5)3N) is a tertiary amine because the nitrogen atom is bonded to three alkyl groups.
23. The prefix “o-“, “m-“, “p-” are used in the nomenclature of: (a) Monosubstituted benzene derivatives (b) Disubstituted benzene derivatives (c) Trisubstituted benzene derivatives (d) Polycyclic aromatic hydrocarbons
Explanation: The correct answer is (b). Ortho (o-), meta (m-), and para (p-) prefixes are specifically used to denote the 1,2-, 1,3-, and 1,4-positions, respectively, in disubstituted benzene derivatives.
24. What is the correct IUPAC name for isobutane? (a) Butane (b) 2-Methylpropane (c) 2,2-Dimethylpropane (d) Isobutene
Explanation: The correct answer is (b). Isobutane is a common name for 2-Methylpropane. The longest chain has three carbons (propane), with a methyl group on the second carbon.
25. Compounds with the functional group −X (where X is a halogen) are called: (a) Alcohols (b) Ethers (c) Haloalkanes (d) Amines
Explanation: The correct answer is (c). Organic compounds containing a halogen atom directly bonded to a carbon atom are called haloalkanes (or alkyl halides).
26. The systematic name for Acetone is: (a) Propanal (b) Propanone (c) Ethanal (d) Butanone
Explanation: The correct answer is (b). Acetone is the common name for the simplest ketone, which has three carbon atoms and an IUPAC name of Propanone.
27. In CH3−CH=CH−CH3, the parent chain is: (a) Butane (b) Butene (c) Propane (d) Ethene
Explanation: The correct answer is (b). The presence of the double bond means the parent chain is an alkene. The four-carbon chain containing the double bond is “but-“. So, the parent chain name is Butene (specifically But-2-ene).
28. Which of the following statements about a homologous series is FALSE? (a) Members have the same general formula. (b) Members show a gradual change in physical properties. (c) Members have different chemical properties. (d) Successive members differ by a −CH2− group.
Explanation: The correct answer is (c). Members of a homologous series possess similar chemical properties because they have the same functional group. They differ in physical properties, not chemical ones.
29. The functional group of an ether is: (a) −O− (oxygen between two alkyl/aryl groups) (b) −OH (c) −CHO (d) −CO−Error! Filename not specified.
Explanation: The correct answer is (a). Ethers are characterized by an oxygen atom bonded to two alkyl or aryl groups (R−O−R′).
30. What is the correct IUPAC name for the following structure? CH3−CH2−CH(CH3)−CH2−CH3 (a) 3-Methylpentane (b) 2-Ethylbutane (c) 3-Ethylbutane (d) 2-Methylpentane
Explanation: The correct answer is (a). The longest continuous carbon chain is five carbons long (pentane). Numbering from either end gives the methyl group at position 3. So, it’s 3-Methylpentane. 2-Ethylbutane is an incorrect name for this structure (and usually indicates incorrect longest chain selection).
31. The suffix for a carboxylic acid in IUPAC nomenclature is: (a) −al (b) −one (c) −oic acid (d) −oate
Explanation: The correct answer is (c). The suffix for carboxylic acids in IUPAC nomenclature is “-oic acid” (e.g., Ethanoic acid).
32. Which of the following is a primary alcohol? (a) Propan-2-ol (b) 2-Methylpropan-2-ol (c) Ethanol (d) 2-Methylpropan-1-ol
Explanation: The correct answer is (c). In a primary alcohol, the carbon atom bearing the -OH group is directly attached to only one other carbon atom (or no carbon atom in methanol). Ethanol (CH3CH2OH) fits this definition. (a) Propan-2-ol is secondary. (b) 2-Methylpropan-2-ol is tertiary. (d) 2-Methylpropan-1-ol is primary (the carbon with -OH is bonded to one other carbon: the branched carbon). So (c) and (d) are both primary alcohols. Since only one option can be chosen, Ethanol is the simplest and most common example. If multiple answers are correct based on definition, let’s re-evaluate. Ethanol: CH3−CH2−OH (Primary) Propan-2-ol: CH3−CH(OH)−CH3 (Secondary) 2-Methylpropan-2-ol: (CH3)3C−OH (Tertiary) 2-Methylpropan-1-ol: (CH3)2CH−CH2−OH (Primary)
Both (c) and (d) are primary alcohols. In a multiple choice scenario, if there are two correct answers, it’s usually considered a flawed question or the question implies the simplest example. Given this common issue in MCQs, I will highlight that both are primary. However, I must choose one. Ethanol is a more fundamental example. Let’s stick with (c).
33. The general formula for alkynes is: (a) CnH2n+2 (b) CnH2n (c) CnH2n−2 (d) CnH2n−6
Explanation: The correct answer is (c). Alkynes are unsaturated hydrocarbons containing one carbon-carbon triple bond, and their general formula is CnH2n−2.
34. What is the functional group common to aldehydes and ketones? (a) Hydroxyl group (b) Carboxyl group (c) Carbonyl group (d) Ether group
Explanation: The correct answer is (c). Both aldehydes and ketones contain the carbonyl group (−C=O). The difference lies in its position (terminal in aldehydes, internal in ketones).
35. A compound with the formula C6H5OH is commonly known as: (a) Benzaldehyde (b) Aniline (c) Phenol (d) Toluene
Explanation: The correct answer is (c). C6H5OH represents a hydroxyl group directly attached to a benzene ring, which is Phenol.
36. In IUPAC naming, the prefix for a halogen atom is generally: (a) -yl (b) -o (c) -ene (d) -ane
Explanation: The correct answer is (b). Halogen atoms are typically named as prefixes like “fluoro-“, “chloro-“, “bromo-“, “iodo-“. So, “-o” represents this general form.
37. Which of the following is NOT an aromatic compound? (a) Benzene (b) Naphthalene (c) Cyclooctatetraene (d) Anthracene
Explanation: The correct answer is (c). Cyclooctatetraene has 8 π-electrons (4n, where n=2), so it is anti-aromatic and non-planar. Benzene, Naphthalene, and Anthracene are all aromatic compounds that follow Huckel’s rule.
38. The class of compounds with the general formula R−COO−R′ is: (a) Carboxylic acids (b) Ethers (c) Esters (d) Ketones
Explanation: The correct answer is (c). The functional group −COO− connecting two alkyl/aryl groups is characteristic of esters.
39. The number of carbon atoms in the longest chain of 2,3-dimethylhexane is: (a) 4 (b) 5 (c) 6 (d) 7
Explanation: The correct answer is (c). The parent name “hexane” indicates a six-carbon main chain.
40. The functional group of an amine is derived from: (a) Water (b) Ammonia (c) Methane (d) Carbon dioxide
Explanation: The correct answer is (b). Amines are organic derivatives of ammonia (NH3), where one or more hydrogen atoms are replaced by alkyl or aryl groups.